The Fine-Tuning Argument for Embryo-Endometrial Synchrony
Is It Likely That Hormonal Contraceptive Induced Desynchronization Will Be Overcome During Pregnancy?
THE CASE FOR EMBRYO-ENDOMETRIAL SYNCHRONY
It is widely accepted in the research community that a successful pregnancy and birth requires embryo-endometrial (E/E) synchrony. Lack of progress improving in-vitro fertilization (IVF) outcomes reinforces that pregnancy is a finely-tuned process that is difficult to re-create successfully in the lab.
“Despite the increasing use of IVF and related assisted reproductive technologies worldwide, success rates are still modest, with only around 25–30% of cycles resulting in a live birth (de Mouzon et al., 2020)”1 (McMahon et al., 2024)
The nature of intricate and interrelated events occurring throughout pregnancy could well be described as a symphony orchestra in synchrony where musicians playing out-of-tune or out-of-time could have disastrous effects.
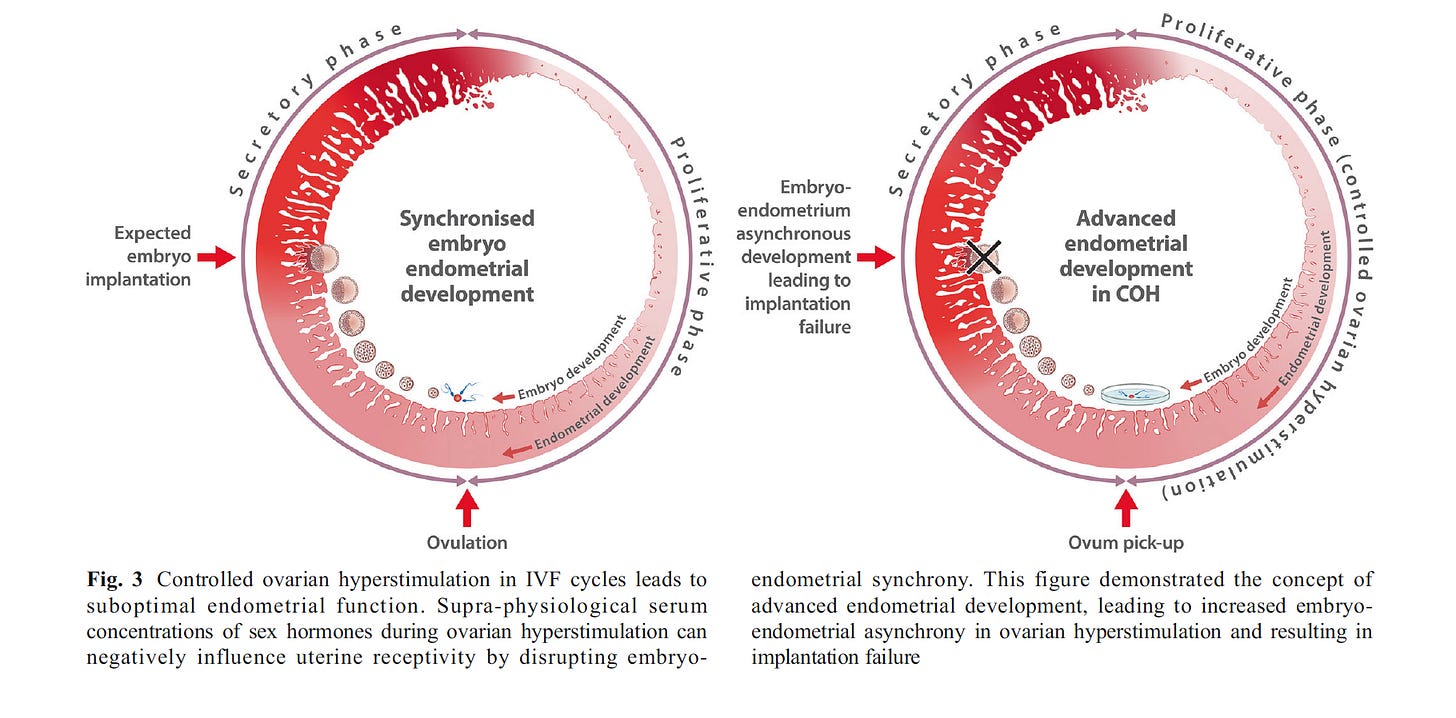
Figure 1 (Figure 3 from the source) is from an article titled, “What is the Contribution of Embryo-endometrial Asynchrony to Implantation Failure?” by Teh, McBain, and Rogers in 2016.2 It illustrates the asynchronization common in controlled ovarian hyperstimulation protocols used during IVF that leads to implantation failure.
Figure 1 above depicts a normally growing endometrium that is mis-timed with the fetal embryo transfer. If timing is off by more than +/- 1.5 days of synchrony, success rates are reduced.
In a similar manner, hormonal contraceptive (HC) use leaves a woman with a desynchronized system in the event of pregnancy but is further confounded by an underdeveloped endometrium.
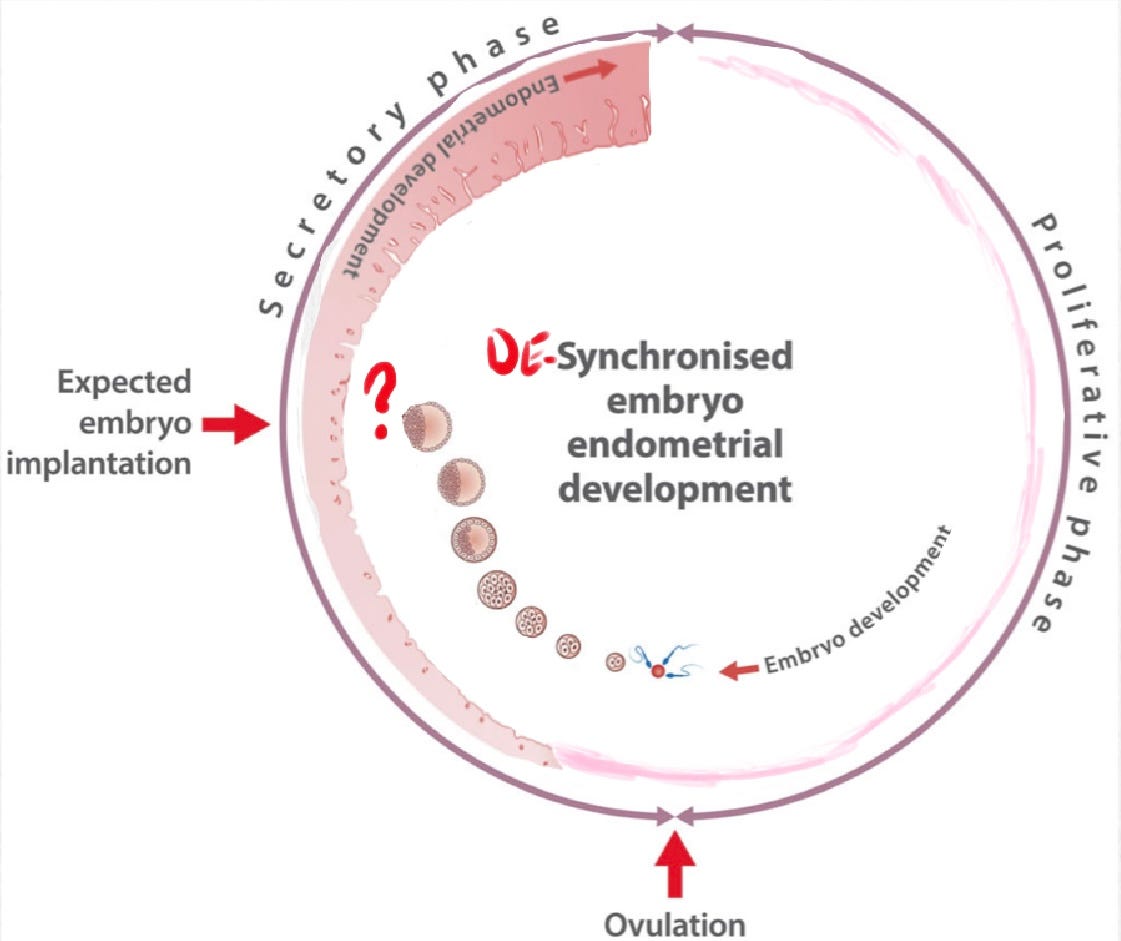
Synchronization requires not only proper timing of events, but also proper preparation and functioning of processes. Poor timing and disruption of fetal-maternal communication pathways increases risk of pregnancy loss.
Early pregnancy loss was once considered a “black box” of knowledge3 (Macklon, 2002). But we are learning more and more:
“Once considered a black box, our understanding of the mechanisms that control human embryo implantation is accelerating rapidly, aided by powerful new technologies including single-cell ‘omics’, spatial transcriptomics, blastoids, organoids and assembloids.” (Muter, 2023)
While we might not yet know all the reasons pregnancies fail, it is universally acknowledged that a successful, normal live-birth outcome requires near perfect conditions. By “near perfect” I mean a high level of embryo-endometrial synchrony which requires normal hormone levels and timing throughout the entire pregnancy process. This includes the proliferative phase (pre-fertilization) and the luteal phase (post-fertilization).
Consider the following research from multiple fields including endocrinology, infertility, and IVF, which emphasize carefully orchestrated synchronization and delicate communication signaling processes involved in menstruation and pregnancy:
“Ovulation follows a carefully orchestrated series of neuroendocrine and intrafollicular events.”4 (Devoto, Kohen, Munoz, Struass, 2009, S19)
“The synchronized development of a viable embryo and a receptive endometrium is critical for successful implantation to take place.”5 (Teh, McBain, Rogers, 2016, p. 1419)
“To produce a viable normal embryo, a sequence of perfectly linked processes must occur in the post-menarchal ovary.”6 (Devesa, Caicedo, 2019, p. 6)
“The formation of a functional corpus luteum relies on the appropriate proliferation and differentiation of both granulosa and theca cells. Any disruption in the crosstalk between granulosa and theca cell or differentiation of these cells types alters lineages and gene expression profiles that could negatively impact luteinization and progesterone production.”7 (Abedel-Majed, Romereim, Davis , Cupp, 2019, p. 11)
“A specific hormone signaling sequence is involved in each phase and stimulates the endometrium to first proliferate and then transform to a receptive state.”8 (Gao et al., 2019, p. 546)
“…the mechanisms driving granulosa cell (GC) progesterone production are much more intricate than previously recognized, with both local and systemic factors interacting to promote successful GC function and physiological fine-tuning of progesterone levels.”9 (DeWitt, Whirledge, Kallen, 2020, p. 2)
“A coordinated sequence of events must occur in order to establish and successfully maintain a healthy pregnancy. Synchrony between the development of the early embryo and establishment of a receptive endometrium is necessary to allow implantation and subsequent progression of pregnancy.”10 (Tal, Hugh, Taylor, 2021)
“LH and FSH production and activity depend on the precise orchestration of numerous elements of the hypothalamic–pituitary–gonadal (HPG) axis...”11 (Bosch et al., 2021, p. 1470)
“Before successful implantation of a blastocyst in the pre-decidualized endometrium, morphological and biochemical changes of both the embryo and the endometrium must be in synchrony.”12 (Bulletti, Bulletti, Sciorio, Guido, 2022, p. 1)
“While embryo quality is a central determinant of implantation and pregnancy success, temporally coordinated differentiation of endometrial stromal cells into DSCs to attain uterine receptivity, and a synchronized cross-talk between maternal and embryonic tissues are crucial for successful implantation”13 (Abuwala, Tal, 2021, p. 6)
“While embryo development and endometrial preparation are concurrent yet independent processes, their synchronization is critical to the success of embryo apposition, adhesion, invasion, and further ongoing pregnancy….When synchrony is lost or receptivity is not achieved, the consequence is early pregnancy loss or infertility….Embryo implantation requires a receptive endometrium, a functional, normally developing embryo, and synchronized embryo-endometrial cross-talk.”14 (Blanco-Breindel, Singh, Kahn, 2023)
“The process of implantation involves a complex sequence of cellular steps that must progress correctly for pregnancy to occur.”15 (Hull, Robertson, 2023)
“Successful implantation requires the exquisitely coordinated migration and invasion of trophoblast cells from the outer capsule of the blastocyst into the endometrium.”16 (Benagiano,Mancuso, Guo, Renzo, 2023, p. 8)
“The endometrium undergoes a complex series of changes during the menstrual cycle in preparation for embryo implantation. This process requires precise coordination between the embryo and the endometrium [8]. Any disruption can lead to RPL or RIF”17 (Braun et al., 2023, p. 2)
“It has been discovered that the complex and delicate dialogs between trophoblasts and DICs [decidual immune cells] are the key link in driving the establishment and maintenance of maternal–fetal immunotolerance.”18 (Yao, Ye, Chen, Zhang, Cai, & Zheng, 2024, p. 3) [addition mine]
In summary, according to the research, a healthy birth requires:
synchronized embryo-endometrial crosstalk
sequence of perfectly linked processes
no disruption in embryo-endometrial crosstalk
specific hormone signal sequencing
fine tuning of hormone levels
intricate, finely-tuned mechanisms
precise orchestration
complex and delicate dialogues
complex sequence of cellular steps that must progress correctly
Protestant hedged contraceptionists have faith that in the event a woman gets pregnant while on HC, a system that has been chronically broken and desynchronized for months or years can be rejuvenated and re-synchronized in about 6-10 days (the time from fertilization to implantation) while the woman is presumably still taking HC since pregnancy recognition may be difficult at such an early stage.
One aspect of embryo-endometrial synchrony is the proper development of the follicle prior to ovulation and the synchronization of egg release, embryo development, and endometrial preparation.
This means that synchronization in pregnancy is not an entirely linear, event-driven phenomenon. Concurrent cyclic processes are happening throughout the body for the duration of the menstrual cycle.
Historically, political questions surrounding the effects of HC have been somewhat linear— before or after fertilization, before or after implantation—as if new and completely independent circumstances are presented in each phase.
But the phases are interconnected and synchronized from the start. For instance, HC seems to be affecting follicle development prior to ovulation…(See more)
McMahon, C., Hammarberg, K., Lensen, S., Wang, R., Mol, B. W., & Vollenhoven, B. J. N. (2024). What do women undergoing in vitro fertilization (IVF) understand about their chance of IVF success?. Human reproduction (Oxford, England), 39(1), 130–138. https://doi.org/10.1093/humrep/dead239
Teh, W. T., McBain, J., & Rogers, P. (2016). What is the contribution of embryo-endometrial asynchrony to implantation failure?. Journal of assisted reproduction and genetics, 33(11), 1419–1430. https://doi.org/10.1007/s10815-016-0773-6
Macklon, N. S., Geraedts, J. P., & Fauser, B. C. (2002). Conception to ongoing pregnancy: the 'black box' of early pregnancy loss. Human reproduction update, 8(4), 333–343. https://doi.org/10.1093/humupd/8.4.333
Devoto, L., Kohen, P., Muñoz, A., & Strauss, J. F., 3rd (2009). Human corpus luteum physiology and the luteal-phase dysfunction associated with ovarian stimulation. Reproductive biomedicine online, 18 Suppl 2, 19–24. https://doi.org/10.1016/s1472-6483(10)60444-0
Teh, W. T., McBain, J., & Rogers, P. (2016). What is the contribution of embryo-endometrial asynchrony to implantation failure?. Journal of assisted reproduction and genetics, 33(11), 1419–1430. https://doi.org/10.1007/s10815-016-0773-6
Devesa, J., & Caicedo, D. (2019). The Role of Growth Hormone on Ovarian Functioning and Ovarian Angiogenesis. Frontiers in endocrinology, 10, 450. https://doi.org/10.3389/fendo.2019.00450
Abedel-Majed, M. A., Romereim, S. M., Davis, J. S., & Cupp, A. S. (2019). Perturbations in Lineage Specification of Granulosa and Theca Cells May Alter Corpus Luteum Formation and Function. Frontiers in endocrinology, 10, 832. https://doi.org/10.3389/fendo.2019.00832
Gao, M., Cao, C., Zhang, X., Tang, F., Zhao, L., Luo, S., & Li, L. (2019). Abnormal expression of estrogen receptor is associated with thin endometrium. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology, 35(6), 544–547. https://doi.org/10.1080/09513590.2018.1554035
DeWitt, N. A., Whirledge, S., & Kallen, A. N. (2020). Updates on molecular and environmental determinants of luteal progesterone production. Molecular and cellular endocrinology, 515, 110930. https://doi.org/10.1016/j.mce.2020.110930
Tal R, Taylor HS. Endocrinology of Pregnancy. [Updated 2021 Mar 18]. In: Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK278962/
Bosch, E., Alviggi, C., Lispi, M., Conforti, A., Hanyaloglu, A. C., Chuderland, D., Simoni, M., Raine-Fenning, N., Crépieux, P., Kol, S., Rochira, V., D'Hooghe, T., & Humaidan, P. (2021). Reduced FSH and LH action: implications for medically assisted reproduction. Human reproduction (Oxford, England), 36(6), 1469–1480. https://doi.org/10.1093/humrep/deab065
Bulletti, C., Bulletti, F. M., Sciorio, R., & Guido, M. (2022). Progesterone: The Key Factor of the Beginning of Life. International journal of molecular sciences, 23(22), 14138. https://doi.org/10.3390/ijms232214138
Abuwala, N., & Tal, R. (2021). Endometrial stem cells: origin, biological function, and therapeutic applications for reproductive disorders. Current opinion in obstetrics & gynecology, 33(3), 232–240. https://doi.org/10.1097/GCO.0000000000000702
Blanco-Breindel MF, Singh M, Kahn J. Endometrial Receptivity. [Updated 2023 Jun 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK587449/
Hull, Louise, Robertson, Sarah, (2023, May 30), Trying for a baby? What you need to know about a vital part of your womb (and how to look after it, theconversation.com, https://theconversation.com/trying-for-a-baby-what-you-need-to-know-about-a-vital-part-of-your-womb-and-how-to-look-after-it-202854
Benagiano, G., Mancuso, S., Guo, S. W., & Di Renzo, G. C. (2023). Events Leading to the Establishment of Pregnancy and Placental Formation: The Need to Fine-Tune the Nomenclature on Pregnancy and Gestation. International journal of molecular sciences, 24(20), 15420. https://doi.org/10.3390/ijms242015420
Braun, A. S., Vomstein, K., Reiser, E., Tollinger, S., Kyvelidou, C., Feil, K., & Toth, B. (2023). NK and T Cell Subtypes in the Endometrium of Patients with Recurrent Pregnancy Loss and Recurrent Implantation Failure: Implications for Pregnancy Success. Journal of clinical medicine, 12(17), 5585. https://doi.org/10.3390/jcm12175585
Yao, Y., Ye, Y., Chen, J., Zhang, M., Cai, X., & Zheng, C. (2024). Maternal-fetal immunity and recurrent spontaneous abortion. American journal of reproductive immunology (New York, N.Y. : 1989), 91(5), e13859. https://doi.org/10.1111/aji.13859